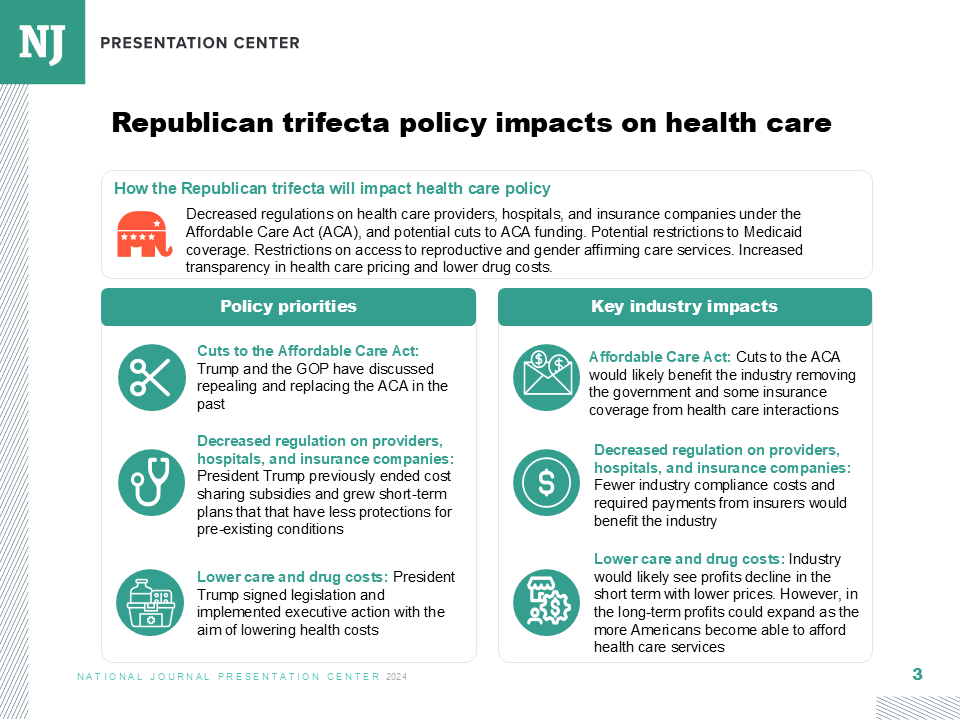
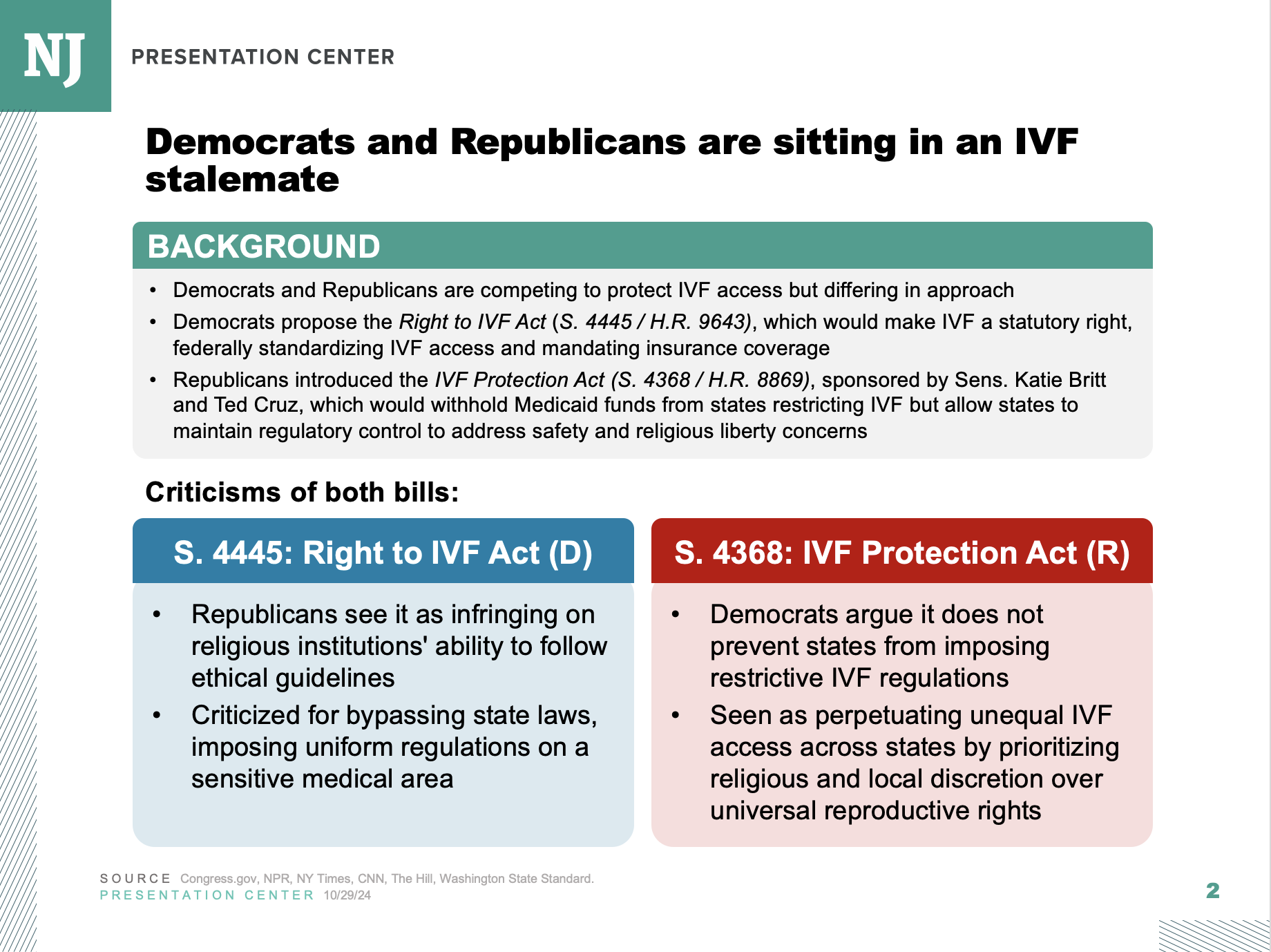
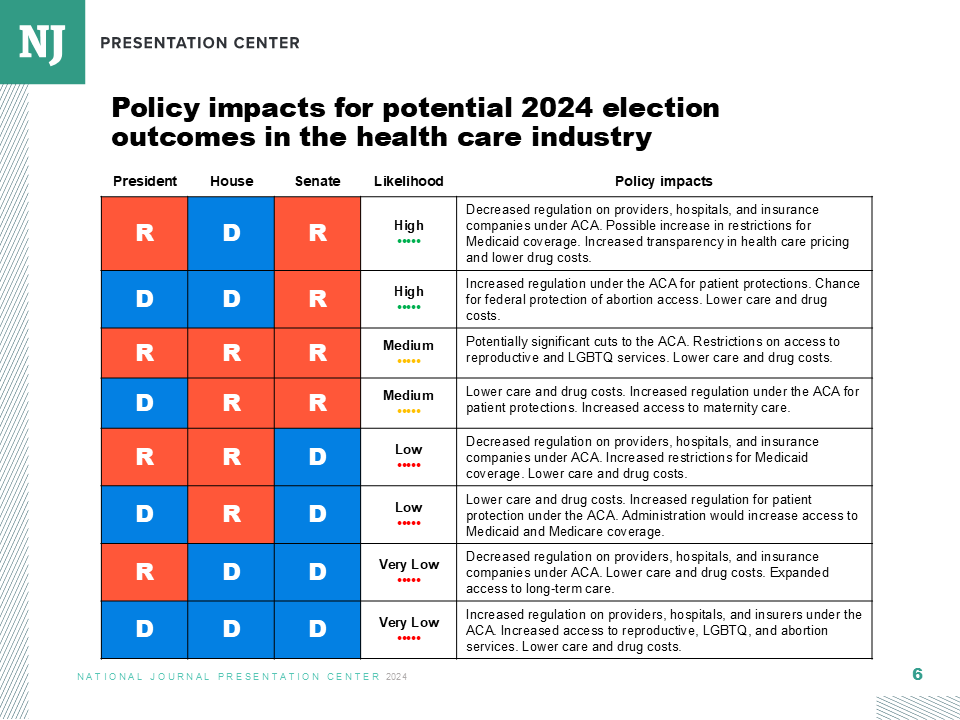
Health and Human Services Secretary Robert F. Kennedy Jr. has been systematically overhauling the U.S. vaccine system—and his efforts could soon turn to expanding resources for those who claim to be injured from inoculation.
In a leaked policy proposal submitted to the White House by HHS, the department indicates the National Institutes of Health's National Institute of Allergy and Infectious Diseases will investigate “vaccine injuries with improved data collection and analysis.” The department also plans to open a new vaccine research program at the NIH Clinical Center, which could expand to centers around the country.
Just last month, Kennedy vowed to overhaul the Vaccine Injury Compensation Program, which was created in the 1980s to give victims who suffer unusual but serious side effects from vaccines compensation without directly targeting vaccine makers.
The secretary scorned the program as “broken,” claiming it has strayed from its congressionally approved mission by prioritizing the solvency of the fund rather than compensating victims. In a recent interview with Tucker Carlson, Kennedy said he has assigned a team to explore how to broaden the population of people who can receive funds from the program.
Expanding compensation to a wider range of people could threaten to bankrupt the program, and it could result in more civil lawsuits directed at vaccine manufacturers. If vaccine producers are forced to pay out larger settlements—or are simply worried about the threat of litigation—it could drive them out of the market, as happened in the 1980s before the VICP program was created.
“If you add a common disorder like autism [to the list of injuries covered by VICP], which is reported to occur in one in 31 children in this country, I think that could break the program,” said Paul Offit, director of the Vaccine Education Center at the Children’s Hospital of Philadelphia.
Before VICP’s creation in 1986, pharmaceutical companies faced hundreds of lawsuits dating back to the 1970s from petitioners related to the DPT vaccine, which protects children from diphtheria, tetanus, and whooping cough.
The lawsuits alleged that those who received the vaccine were experiencing side effects such as seizures that led to disabilities. As manufacturers paid out settlements, a number of vaccine makers began leaving the U.S. market, resulting in vaccine shortages and a delay of shots for children.
Through VICP, those suffering adverse effects can file a petition through a no-fault system that provides compensation to victims, while protecting vaccine makers from being found responsible for negligence in the traditional legal system. HHS staff review the petition, determine if it meets medical criteria, and make a preliminary recommendation. The Justice Department then develops a report that includes the recommendation and legal analysis, which is presented to a special master.
The special master is the person who decides if the petitioner should be compensated, often after a hearing at which both parties can present evidence. The court can order HHS to award compensation—and possibly pay the petitioner’s legal fees and costs, even if the petition is dismissed. In some cases, victims can be compensated for injuries that meet the criteria in the program’s injury table even if their injuries were not proven to stem from vaccines.
During Kennedy’s interview with Carlson, the secretary said his team is looking at ways to extend the statute of limitations dictating when a claim can be filed. Currently, a petition must be filed within two years if death from vaccination is alleged to have occurred, or three years if injury is alleged. Kennedy argued that “a lot of people don’t discover their injuries” within three years.
Another possibility is that Kennedy will add autism or other disorders to the Vaccine Injury Table, which determines the medical criteria to receive compensation. In public statements through the years, Kennedy has repeatedly suggested that autism may be caused by vaccines.
During the interview, Kennedy indicated that he’s looking beyond autism, also mentioning diabetes, rheumatoid arthritis, ADHD, speech delay, language delay, narcolepsy, and other disorders as potentially linked to vaccines. These disorders affect a large part of the U.S. population, and the changes could make the fund far more accessible—while also possibly draining it.
In addition, Kennedy has the power to change which vaccines are covered by the program, and dropping routine inoculations from the list of covered shots could leave manufacturers vulnerable to the same type of lawsuits they faced in the 1980s.
“He wants to make vaccines less available, less affordable, and more feared. And that’s all this is about,” Offit said.
Last week, Kennedy revived the Task Force on Safer Childhood Vaccines, a long-defunct panel that handled vaccine safety oversight. The task force is expected to work closely with the Advisory Commission on Childhood Vaccines to produce recommendations. ACCV advises HHS on implementation of the VICP, including recommending changes to the Vaccine Injury Table.
Other moves by Kennedy have systematically chipped away at the country’s vaccine infrastructure.
In May, Kennedy bucked the normal process for vaccine review and approval when he overruled the previous recommendation of the Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices that healthy pregnant women and children should receive the COVID-19 vaccine. Kennedy, along with many anti-vaccine groups, has long argued that the COVID-19 vaccine is unsafe and ineffective.
In June, the secretary fired all 17 members of ACIP, replacing many of them with vaccine skeptics. The new ACIP panel has since met to offer recommendations to the CDC—and its current focus underscores much of Kennedy’s past rhetoric on vaccines. One of the panel’s first votes was to eliminate thimerosal, a mercury-based preservative, from all flu vaccines. Kennedy has long criticized the use of thimerosal in vaccines, claiming without credible evidence that the preservative could cause neurological issues such as autism.
The panel, which is expected to meet again in August or September, is likely to review childhood vaccines and shots that haven’t been studied in more than seven years, according to committee chair Martin Kulldorff. In June, Kulldorff stated that the panel might consider advising against the MMRV combination shot, which protects children under 4 years of age against measles and chickenpox.
Earlier this month, Kennedy announced plans to cancel $500 million in mRNA vaccine development projects. Under the first Trump administration’s “Project Warp Speed,” mRNA technology was used to quickly develop the COVID-19 vaccine. The vaccine has been credited with saving millions of lives during the pandemic, but skeptics, including Kennedy, have cast doubt on the safety and effectiveness of the technology.
Authorizations of certain vaccines through the Food and Drug Administration are also under threat.
According to recent reports, the FDA is considering revoking its authorization of Pfizer’s COVID-19 vaccine for healthy children under the age of 5. This comes after the agency granted full approval to Moderna’s COVID-19 vaccine for children, but only for groups that are at an increased risk from the disease. The Novavax COVID-19 shot has never been approved for children under 12.
At NIH, the Trump administration has targeted grants aimed at studying vaccine hesitancy. According to NPR reporting, more than 40 grants related to vaccine hesitancy and ways to increase immunization levels have been canceled.